Many electronic appliances are at the threshold of a revolution that began with the discovery of polymeric conductors in the 1970s. Polymeric materials, which have historically been classified exclusively as electrical insulators, are now finding varied applications as both conductors and semiconductors. Expensive ceramic semiconductors that are brittle and difficult to pattern have historically been the driving force of the digital age for the last fifty years. But now a combination of properties exist today in polymers that are making many previously impossible appliances a reality.
Within a very short time organic conductors have been developed with the conductivity of metals such as copper, while organic electronics has evolved photoelectric cells, diodes, light emitting diodes, lasers and transistors. The result is that a class of plastic materials referred to as conjugated polymers are fast displacing traditional materials such as natural polymers (e.g. wood), metals, ceramics and glass in many applications owing to the combination of their physical/mechanical properties (light weight combined with physical strength) and ease of processibility (the ability to mould the shape of plastic materials or extrude into sheet and rod through a die).
What this means is that OLEDs can be deployed in a wide range of electronic devices and can be used extensively throughout any given device. For monitors and mobile phones you can check out OLED TVs for sale. Active components of displays can be polymers, substrates can be polymers, logical electronics can be polymers, and so on. In the years ahead OLEDs will see applications in personal computers, cell phones, televisions, general wide area lighting, signs, billboards, communications and any of a number of information appliances.
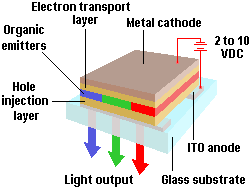
The basic OLED cell structure consists of a stack of thin organic layers sandwiched between a transparent anode and a metallic cathode. The organic layers comprise a hole-injection layer, a hole-transport layer, an emissive layer and an electron-transport layer. When an appropriate voltage (typically a few volts) is applied to the cell, the injected positive and negative charges recombine in the emissive layer to produce light (electroluminescence). The structure of the organic layers and the choice of anode and cathode are designed to maximise the recombination process in the emissive layer, thus maximising the light output from the OLED device. Both the electroluminescent efficiency and control of colour output can be significantly enhanced by doping the emissive layer with a small amount of highly fluorescent molecules.
To drive a passive-matrix OLED display, electrical current is passed through selected pixels by applying a voltage to the corresponding rows and columns from drivers attached to each row and column. An external controller circuit provides the necessary input power, video data signal and multiplex switches. Data signal is generally supplied to the column lines and synchronised to the scanning of the row lines. When a particular row is selected, the column and row data lines determine which pixels are lit. A video output is thus displayed on the panel by scanning through all the rows successively in a frame time, typically 1/60th of a second.
In contrast to the passive-matrix OLED display, an active-matrix OLED has an integrated electronic backplane as its substrate, comprising at least two transistors for every pixel. These transistors are in turn connected to perpendicular anode and cathode lines, and are able to hold any active pixels in the on state until the next scan period. Because of their additional parts, active matrix displays are more complex to build, though they offer a brighter and more defined images than their less costly passive matrix counterparts.
Since an OLED is an emissive device, the display aperture factor is not critical, unlike LCD displays where light must pass through an aperture. Consequently there are no intrinsic limitations to the pixel count, resolution, or size of an active-matrix OLED display. Also, in an OLED display a defective pixel produces only a dark defect, which is considered to be much less objectionable than an LCD bright point defect. Furthermore, constant-current drivers can be built directly on the substrate, thus eliminating the need for high-density (and expensive) interconnects and peripheral drivers.
A large number of research groups around the world have become engaged in OLED research and development, with universities, large corporations and government labs all represented as well as entrepreneurial start-ups. Much of their efforts are directed towards solving the two main Achilles heels of OLED development: poor efficiency and short lifetime. However, it is expected that these problems will be overcome and that full-colour OLED-based displays will have replaced active matrix LCDs as the market dominant display technology by around 2010.
OLEDs are classified according to the size of molecules which make up the materials of which they are composed, and two principal technology branches have emerged in recent years – using relatively large molecules known as Light-Emitting Polymers (LEPs) and using relatively small molecules (even monomers) known as Small Molecule Organic Light Emitting Diodes (SMOLEDs). Both generate light when an electric current is applied to a thin film section. Electrons and holes are formed, which recombine through two types of states: the singlet state, which emits light, and the triplet state, which does not. The efficiency of OLEDs is limited by the ratio of light-emitting singlet states to non-emitting triplet states.
Some research results have indicated that for SMOLEDs the formation of the singlet state is three times less likely than the triplet state, placing an upper limit on device efficiency of 25%. As a consequence significant research and development effort is necessary to improve SMOLED performance, for example, by adding phosphors and/or utilising triplet state conversion to extend singlet emission. By contrast, the same research has indicated that the singlet/triplet ratio in LEPs could be greater than one – more than twice the previously estimated maximum theoretical efficiency – indicating that very high efficiency LEPs are achievable without the addition of phosphors.
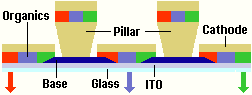
Another disadvantage of the SMOLED method is that the production of quality films requires vapour sublimation in a vacuum chamber whilst LEPs may be dissolved and applied using standard solvent coating techniques.
- VA – Vertically Aligned LCD Monitors
- What in the LCD is IPS!?
- ThinCRT Flat Panels
- TFT LCD Monitors
- LCD Resolutions and Picture Scaling
- Liquid Crystal Light Polarisation in LCD Monitors
- Polysilicon Flat Panels
- Plasma Flat Panels
- PALCD Flat Panels
- OLED Flat Panels
- MVA – Multi-domain Vertical Alignment in LCD Monitors
- LEP Flat Panels
- LED Flat Panels
- LCD – Liquid Crystal Displays
- IPS – In-Plane Switching LCD Monitors
- HAD Flat Panels
- Flat Panel Feature Comparisons
- FED Flat Panels
- Digital Flat Panels
- DSTN LCD monitors
- Creating Colour in LCD Displays
- Flat Panel ALiS Technology